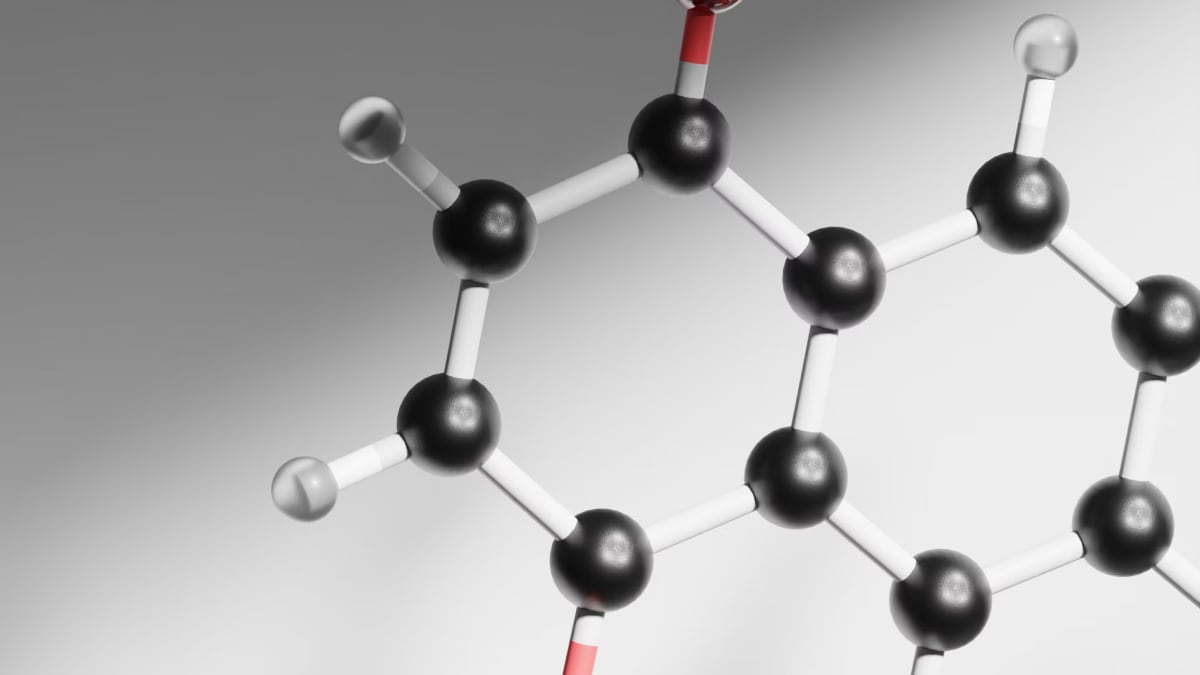
A recent discovery by UCLA scientists has challenged a century-old principle in organic chemistry, reshaping fundamental knowledge and broadening possibilities for pharmaceutical research. Researchers who were led by Professor Neil Garg, have found a way to synthesize and stabilize anti-Bredt olefins (ABOs), molecular structures. These structures were long considered too unstable to exist. This accomplishment dismantles Bredt's rule—a restriction from 1924 that has influenced molecular design for decades—allowing chemists to explore new chemical reactions in drug development.
Bredt's Rule and Its Historical Significance
Established by chemist Julius Bredt nearly a hundred years ago, Bredt's rule asserts that double bonds cannot exist at the bridgehead position in certain molecules, as this structure would disrupt molecular stability. Bredt's rule has held strong for decades, barring chemists from designing certain types of synthetic compounds. Given that double bonds or phenols, are widely used in pharmaceuticals, this limitation has affected the landscape of drug design by restricting the diversity of possible molecular structures.
How UCLA Researchers Achieved the Impossible
In a paper published in Science, Garg and his team reveal a method to create ABOs by treating molecules known as silyl (pseudo)halides with a fluoride source, which sparks an elimination reaction, leading to ABO formation. To handle the instability of ABOs, the team introduced a trapping agent to stabilize the molecules, allowing them to isolate practical reaction products. This approach provides chemists with a controlled way to work with ABOs, opening up pathways to design unique compounds with real-world applications.
Implications for the Future of Drug Discovery
According to Garg, the pharmaceutical industry has a strong interest in generating 3D structures like those which are now achievable with ABOs. It could be critical for discovering novel drugs. “For over a century, chemists have avoided anti-Bredt olefins, believing them impossible to work with,” Garg said, highlighting the potential of these newly accessible compounds for drug innovation. Co-author and computational chemistry expert Professor Ken Houk's collaboration also helped elucidate the potential of these compounds in practical applications.
This finding invites chemists to rethink molecular rules as flexible guidelines rather than fixed laws, could catalyse a wave of innovation in synthetic chemistry and pharmaceutical development.
For the latest tech news and reviews, follow Gadgets 360 on X, Facebook, WhatsApp, threads and Google NewsFor the latest videos on gadgets and tech, subscribe to our YouTube channelIf you want to know everything about top influencers, follow our in-house Who'sThat360 on Instagram and YouTube,

Ajayante Randam Moshanam to Premiere on Disney+ Hotstar on November 8
Realme GT 7 Pro With Snapdragon 8 Elite SoC, 6,500mAh Battery Launched: Price, Specifications